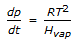Chemical Engineering :: Chemical Engineering Thermodynamics
-
Pick out the wrong statement.
-
Which of the following is clausius-Clayperon equation for vaporisation of an ideal gas under the condition that the molar volume of liquid is negligible compared to that of the vapor ?
-
When a gas is subjected to adiabatic expansion, it gets cooled due to
-
Joule-Thomson effect i.e., a throttling process is a constant __________ process.
-
In the equation, PVn = Constant, if the value of n = 0, then it represents a reversible __________ process.
-
Work done in an adiabatic process between two states depends on the
-
The internal energy of an ideal gas is a function of its __________ only.
-
At constant temperature and pressure, for one mole of a pure substance, the ratio of the free energy to the chemical potential is
-
The change in __________ is equal to the reversible work for compression in steady state flow process under isothermal condition.


 Whatsapp
Whatsapp
 Facebook
Facebook