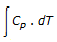Chemical Engineering :: Chemical Engineering Thermodynamics
-
The expression for entropy change given by, ΔS = - nR ln (P2/P1), holds good for
-
With increase in temperature, the internal energy of a substance
-
It is desired to bring about a certain change in the state of a system by performing work on the system under adiabatic conditions.
-
For a constant pressure reversible process, the enthalpy change (ΔH) of the system is


 Whatsapp
Whatsapp
 Facebook
Facebook