Discussion :: Chemical Engineering Thermodynamics
-
Pick out the wrong statement.
|
A.
The chemical potential of a pure substance depends upon the temperature and pressure.
|
|
B.
The chemical potential of a component in a system is directly proportional to the escaping tendency of that component.
|
|
C.
The chemical potential of ith species (μi) in an ideal gas mixture approaches zero as the pressure or mole fraction (xi) tends to be zero at constant temperature.
|
|
D.
The chemical potential of species 'i' in the mixture (μi) is mathematically represented as,
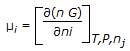 , where, n, ni and nj respectively denote the total number of moles, moles of ith species and all mole numbers except ith species. 'G' is Gibbs molar free energy. , where, n, ni and nj respectively denote the total number of moles, moles of ith species and all mole numbers except ith species. 'G' is Gibbs molar free energy. |
Answer : Option C
Explanation :
No answer description available for this question.
Be The First To Comment


 Whatsapp
Whatsapp
 Facebook
Facebook

