Discussion :: Chemical Reaction Engineering
-
Second order consecutive irreversible reactions
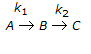 were carried out in a constant volume isothermal batch reactor with different initial feed compositions. Reactor temperature was same in all the cases. In experiments where the ratio of concentration of B to that of A in the initial feed was less than 0.5, the concentration of B increased first, reached a maximum and then declined with time. However, for all experiments where this concentration ratio was 0.5 or above, concentration of B decreased monotonically with time right from the beginning. What is the ratio of the two rate constants (k1/k2)?
were carried out in a constant volume isothermal batch reactor with different initial feed compositions. Reactor temperature was same in all the cases. In experiments where the ratio of concentration of B to that of A in the initial feed was less than 0.5, the concentration of B increased first, reached a maximum and then declined with time. However, for all experiments where this concentration ratio was 0.5 or above, concentration of B decreased monotonically with time right from the beginning. What is the ratio of the two rate constants (k1/k2)?
Answer : Option A
Explanation :
No answer description available for this question.
Be The First To Comment


 Whatsapp
Whatsapp
 Facebook
Facebook

