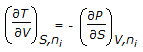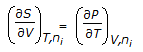Chemical Engineering :: Process Control and Instrumentation
-
In a P-V diagram (for an ideal gas), an isothermal curve will coincide withan adiabatic curve (through a point), when
-
For an ideal gas, the chemical potential is given by
-
Two substances are in equilibrium in a reversible chemical reaction. If the concentration of each substance is doubled, then the value of the equilibrium constant will be
-
In the ammonia synthesis reaction, N2 + 3H2
 2NH3 + 22.4 kcal, the formation of NH3 will be favoured by
2NH3 + 22.4 kcal, the formation of NH3 will be favoured by -
Heat requirement for decomposition of a compound into its elements is __________ that is evolved during the formation of that compound from its elements.
-
The unit of equilibrium constant of a chemical reaction is the same as that of
-
Which of the following equations is obtained on combining 1st and 2nd law of thermodynamics, for a system of constant mass?
-
Fugacity of a component in an ideal gas mixture is euqal to the partial pressure of that component in the mixture. The fugacity of each component in a stable homogeneous solution at contant pressure and temperature __________ as its mole fraction increases.


 Whatsapp
Whatsapp
 Facebook
Facebook