GATE 2017-2018 :: GATE Metallurgical
-
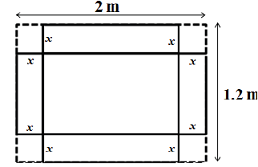
From a 2 m * 1.2 m sheet, squares are cut out from each of the four corners as shown in the figure and then the sides are bent to form an open box. The maximum possible volume (in m3) of the box is __________
- Applying the secant method, the first approximation to the root of f(x) = 1 + ln x + x/2, starting with function values at x = 0.3 and x = 0.4, is_______
- The critical internal crack length (in mm) in a steel having KIc of 45 MPa√m to support a Mode-I stress of 400 MPa is __________
-
Ladle deoxidation of liquid steel is done at 1600°C by adding ferro-aluminium. By assuming Stokes law behaviour, time (in s) required for alumina particles of 50 μm diameter to float to the surface from a depth of 2 m would be__________ [Given: density of steel = 7000 kg/m3, density of alumina = 3650 kg/m3, viscosity of steel = 6 * 10-3 kg/m/s]
-
A steel specimen containing 0.2 wt.% C is carburized in an atmosphere that maintains a carbon content of 1.2 wt.% C at the surface of the specimen. Given: For carbon diffusion in austenite: D0=2.0×10-5 m2/sActivation energy for diffusion, Q=142 kJ/mol
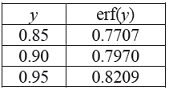 What is the depth (in μm) from the surface of the specimen at which a composition of 0.4 wt.% C is obtained after carburizing at 870°C for 10 h?
What is the depth (in μm) from the surface of the specimen at which a composition of 0.4 wt.% C is obtained after carburizing at 870°C for 10 h? -
A steel specimen containing 0.2 wt.% C is carburized in an atmosphere that maintains a carbon content of 1.2 wt.% C at the surface of the specimen. Given: For carbon diffusion in austenite: D0=2.0×10-5 m2/sActivation energy for diffusion, Q=142 kJ/mol
 How long (in h) will it take to double the depth at which 0.4 wt.% C is reached?
How long (in h) will it take to double the depth at which 0.4 wt.% C is reached? -
Integral enthalpy of mixing (in J/mol) of liquid (Cu, Zn) solution can be approximated by ∆Hmixm = -19250 xCu xZnThe corresponding partial molar enthalpy of mixing (in J/mol) for Cu is
-
Integral enthalpy of mixing (in J/mol) of liquid (Cu, Zn) solution can be approximated by ∆Hmixm = -19250 xCu xZnAssuming regular solution behaviour, the solution parameter (in J/mol) is
-
The density and associated crystallinity for two polypropylene samples are as follows: density, g/cm3 crystallinity, %1.20 501.44 80Density of totally amorphous polypropylene is
-
The density and associated crystallinity for two polypropylene samples are as follows: density, g/cm3 crystallinity, %1.20 501.44 80The percent crystallinity of polypropylene sample having a density of 1.3 g/cm3 is


 Whatsapp
Whatsapp
 Facebook
Facebook


