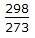Chemical Engineering :: Stoichiometry
-
Which of the following gases will have the- highest kinetic energy per mole at the same pressure & temperature ?
-
What fraction of the total pressure is exerted by oxygen, if equal weights of oxygen and methane are mixed in an empty vessel at 25°C ?
-
On mixing 56 gm of CaO with 63 gm of HNO3, the amount of Ca(NO3)2 formed is __________ gm.
-
The pressure under which liquid and vapor can co-exit at equilibrium is called the __________ vapor pressure.
-
Unrestrained expansion of an ideal gas does not result in its cooling due to the reason that the gas molecules
-
Assuming applicability of ideal gas law, the pure component volume of the vapor in a saturated gas can be calculated from theoretical relationship. The volumetric composition of a vapor saturated gas is independent of the
-
Air at a temperature of 20°C and 750 mm Hg pressure has a relative humidity of 80%. What is its percentage humidity ? Vapour pressure of water at 20°C is 17.5 mm Hg.
-
One mole of methane undergoes complete combustion in a stoichiometric amount of air. The reaction proceeds as CH4 + 2O2 → CO2 + 2H2O. Both the reactants and products are in gas phase. ΔH°298 = - 730 kJ/mole of methane. If the average specific heat of all the gases/vapour is 40 J/mole.K, the maximum temperature rise of the exhaust gases in °C would be approximately equal to
-
As per Kirchoff s equation, the heat of reaction is affected by the
-
In a chemical process, the recycle stream is purged for


 Whatsapp
Whatsapp
 Facebook
Facebook