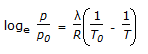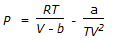Chemical Engineering :: Stoichiometry
-
One kg of saturated steam at 100°C and 1.01325 bar is contained in a rigid walled vessel. It lias a volume of 1.673 m3. It cools to 98°C ; the saturation pressure is 0.943 bar ; one kg of water vapour under these conditions has a volume of 1.789 m3. The amount of water vapour condensed (in kg) is
-
Which of the following holds good for a solution obeying Raoult's law (i.e., an ideal solution) (where, ΔH = heat of mixing, and ΔV = volume change on mixing ) ?
-
Which equation is not an equation of state ?
-
Number of gm moles of solute dissolved in 1 kg of solvent is called its
-
Size range of the colloidals particles is
-
The latent heat of vaporisation
-
Assume that benzene is insoluble in water. The normal boiling points of benzene and water are 80.1 and 100°C respectively. At a pressure of 1 atm, the boiling point of a mixture of benzene and water is
-
Pick out the wrong unit conversion of heat transfer co-efficient.
-
The value of gas constant 'R' is __________ kcal/kg.mole.°C.
-
Diffusion is that property by virtue of which a perfume bottle when opened up in a room, makes the whole room fragrant with its smell. If a perfume 'X' diffuses twice as fast as another perfume 'Y'; what is the molecular weight of 'Y', if the vapor density of gas 'X' is 2 ? Molecular weight of gas 'X' is to be assumed to be 2.


 Whatsapp
Whatsapp
 Facebook
Facebook