GATE 2017-2018 :: GATE Chemistry
-
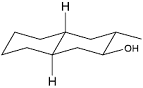
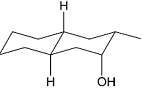
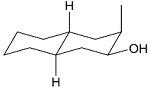
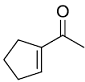
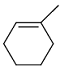
Identify the product from the following reaction
 (9-BBN = 9-Borabicyclo[3¢3¢1]nonane)
(9-BBN = 9-Borabicyclo[3¢3¢1]nonane) -
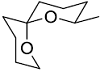
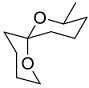
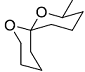
The acid catalyzed cyclization of 5-ketodecan-1,9-diol is given below
 The most predominant spiroketal is
The most predominant spiroketal is - For a face centered cubic lattice, the Miller indices for the first Bragg's peak (smallest Bragg angle) are
-
Consider the following pairs of complexes [CoF(NH3)5]2+ and [Cr(OH2)6]2+[Co(NH3)5 (OH2)]3+ and [Cr(OH2)6]2+[Co(NH3)6]3+ and [Cr(OH2)6]2+[CoI(NH3)5]2+ and [Cr(OH2)6]2+The electron transfer rate will be fastest in the pair
- Hemoglobin is an oxygen carrying protein. The correct statement about oxy-hemoglobin is that
- If a mixture of NaCl, conc. H2SO4 and K2Cr2O7 is heated in a dry test tube, a red vapour (P) is formed. This vapour (P) dissolves in aqueous NaOH to form a yellow solution, which upon treatment with AgNO3 forms a red solid (Q). P and Q are, respectively
-
For the following reaction 2MnO4─ + 5H2C2O4 + 6H+ → 2Mn2+ + 8H2O + 10CO2Eo(MnO4─/Mn2+) = +1.51 V and Eo(CO2/H2C2O4) = ─ 0.49 V.At 298 K, the equilibrium constant is
- The ground states of high-spin octahedral and tetrahedral Co(II) complexes are, respectively
- The INCORRECT statement about Zeise's salt is
|
A.
the metal is low-spin in +3 oxidation state while dioxygen is in O2 ─ form
|
|
B.
the metal is high-spin in +3 oxidation state while dioxygen is in OO2 ─ form
|
|
C.
the metal is low-spin in +3 oxidation state while dioxygen is in neutral form
|
|
D.
the metal is high-spin in +3 oxidation state while dioxygen is in neutral form
|

 .
. .
. .
. .
.
 Whatsapp
Whatsapp
 Facebook
Facebook



 .
. .
. .
. .
.
 .
. .
. .
. .
.