GATE 2017-2018 :: GATE Chemistry
-
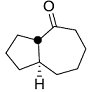
Consider the reaction sequence shown below:
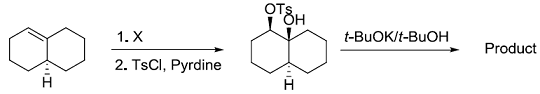 TsCl = p-toluenesulfonyl chlorideThe oxidant X used in step 1 is
TsCl = p-toluenesulfonyl chlorideThe oxidant X used in step 1 is -
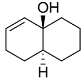
Consider the reaction sequence shown below:
 TsCl = p-toluenesulfonyl chlorideThe product is
TsCl = p-toluenesulfonyl chlorideThe product is -
Consider the E1 reaction of tert-amyl halides from the energy profile given below.
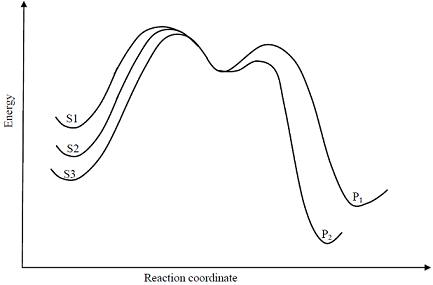
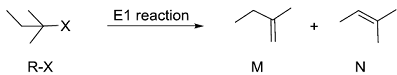 In the above reaction, X = Cl, Br or I. Based on the graph, identify the alkyl halides (R-X) as S1, S2 and S3
In the above reaction, X = Cl, Br or I. Based on the graph, identify the alkyl halides (R-X) as S1, S2 and S3 -
Consider the E1 reaction of tert-amyl halides from the energy profile given below.
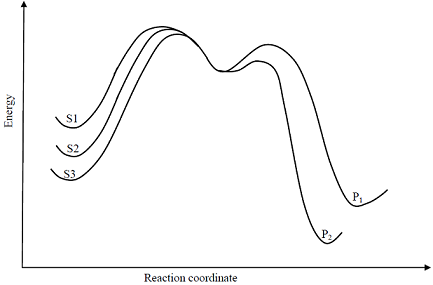
 Identify product P1 and its yield relative to P2
Identify product P1 and its yield relative to P2 -
A 20491 cm─1 laser line was used to excite oxygen molecules (made of 16O only) to obtain the rotational Raman spectrum. The resulting rotational Raman spectrum of oxygen molecule has the first Stokes line at 20479 cm─1.T he rotational constant (usually denoted as B) for the oxygen molecule is
-
A 20491 cm─1 laser line was used to excite oxygen molecules (made of 16O only) to obtain the rotational Raman spectrum. The resulting rotational Raman spectrum of oxygen molecule has the first Stokes line at 20479 cm─1. The next rotational Stokes line is expected at
-
Hückel molecular orbital theory can be applied to the allene radical CH2â•CH─ĊH2The secular determinant (where α , β and E have their usual meanings) is given by
-
Hückel molecular orbital theory can be applied to the allene radical CH2â•CH─ĊH2The possible values of E are
-
One of the parts (A, B, C, D) in the sentence given below contains an ERROR. Which one of the following is INCORRECT? I requested that he should be given the driving test today instead of tomorrow.


 Whatsapp
Whatsapp
 Facebook
Facebook



 .
. .
. .
. .
.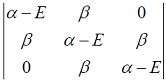 .
.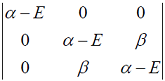 .
.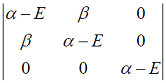 .
. .
.