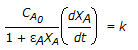Chemical Engineering :: Chemical Reaction Engineering
-
The increase in the rate of reaction with temperature is due to
-
A catalyst loses its activity due to
-
'N' plug flow reactors in series with a total volume 'V' gives the same conversion as a single plug flow reactor of volume 'V' for __________ order reactions.
-
Specific rate constant for a second order reaction
-
For the irreversible elementary reactions in parallel viz
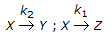 , the rate of disappearance of 'X' is equal to
, the rate of disappearance of 'X' is equal to -
For an isothermal variable volume batch reactor, the following relation is applicable for a first order irreversible reaction.
-
For a zero order chemical reaction, the
-
BET apparatus
-
The excess energy of reactants in a chemical reaction required to dissociate into products is termed as the __________ energy.


 Whatsapp
Whatsapp
 Facebook
Facebook