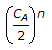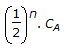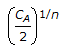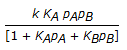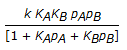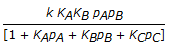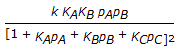Chemical Engineering :: Chemical Reaction Engineering
-
Which of the following resistances is not involved in a gas phase catalytic (gas-solid) reaction ?
-
If the time required to change the concentration of reactant to half its original value is independent of the initial concentration, the order of reaction is
-
A batch adiabatic reactor at an initial temperature of 373°K is being used for the reaction, A
 B. Assume the heat of reaction is - 1kJ/mole at 373°K and heat capacity of both A and B to be constant and equal to 50J/mole.K. The temperature rise after a conversion of 0.5 will be
B. Assume the heat of reaction is - 1kJ/mole at 373°K and heat capacity of both A and B to be constant and equal to 50J/mole.K. The temperature rise after a conversion of 0.5 will be -
If CA is the quantity of reactants initially present, the quantity left after 'n' half periods will be equal to
-
A photochemical reaction is
-
The rate controlling step for the heterogeneous irreversible catalytic reaction A(g) + B(g)
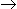 C(g) is the surface reaction of absorbed A with absorbed B to give adsorbed C. The rate expression for this reaction can then be written as (where, KA, KB and KC are the equilibrium constants and is the rate constant of the rate controlling step.)
C(g) is the surface reaction of absorbed A with absorbed B to give adsorbed C. The rate expression for this reaction can then be written as (where, KA, KB and KC are the equilibrium constants and is the rate constant of the rate controlling step.) -
The optimum performance for reactors operating in parallel is obtained when the feed stream is distributed in such a way, that the
-
A catalyst is said to be a negative catalyst, ifit
-
For the non catalytic reaction of particles with surrounding fluid, the same needed to achive the same fractional conversion for particles of different unchanging sizes is proportional to the particle diameter, when the __________ is the controlling resistance.
-
Pure ethanol vapor is fed to a reactor packed with alumina catalyst, at the rate of 100 kmole / hr. The reactor products comprise: ethylene :95 kmole / hr, water vapour: 97.5 k mole / hr and diethyl ether :2.5 kmole/hr. The reactions occuring can be represented by:
C2H5OH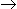 C2H4 + H2O
C2H4 + H2O
2C2H5OH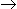 C2H5 - O - C2H5 + H2O
C2H5 - O - C2H5 + H2O
The percent conversion of ethanol in the reactor is


 Whatsapp
Whatsapp
 Facebook
Facebook