Chemical Engineering :: Chemical Reaction Engineering
-
The following half life data are available for the irreversible liquid phase reaction A
 products.
products.
The overall order of reaction is -
A non-catalytic chemical reaction of the type
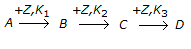 is called a __________ reaction.
is called a __________ reaction. -
The unit of frequency factor in Arhenious equation is
-
With increase in the order of reaction (for all positive reaction orders), the ratio of the volume of mixed reactor to the volume of plug flow reactor (for identical feed composition, flow rate and conversion)
-
Reaction of benzene with chlorine gas to produce tri-chlorobenzene exemplifies a/an __________ reaction.
-
The molecularity and the order of reaction respectively, for the hydrolysis of methyl acetate in presence of acids are
-
The irreversible reaction is a special case of reversible reaction, if the
-
A pollutant P degrades according to first order kinetics. An aqueous stream containing P at 2 kmole/m3 and volumetric flow rate 1m3 /h requires a mixed flow reactor of volume V to bring down the pollutant level to 0.5 kmole/m3 . The inlet concentration of the pollutant is now doubled and the volumetric flow rate is tripled. If the pollutant level is to be brought down to the same level of 0.5 kmole/m3 , the volume of the mixed flow reactor should be increased by a factor of
-
For the liquid phase parallel reactions:
R, rR = K1.CA2; E1 = 80 KJ/mole
S, rs = K1.CA ; E2 = 120 KJ/mole
The desired product is R. A higher selectivity of R will be achieved, if the reaction is conducted at -
In an ideal mixed reactor (at steady state), the


 Whatsapp
Whatsapp
 Facebook
Facebook


