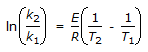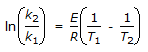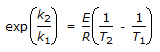Chemical Engineering :: Chemical Reaction Engineering
-
The knowledge of initial concentration and rate constant is necessary to determine the half life time of a reaction of __________ order.
-
Which of the following factors control the design of a fluid-solid reactor ?
-
A Catalyst
-
The synthesis of proteins and metabolism in biological objects occur in the presence of biocatalyst called
-
Pick out the wrong statement.
-
Kinetics of a catalytic reaction can be best studied on a/an __________ reactor.
-
In case of a __________ reactor, the composition in the reactor and at the exit of the reactor is the same.
-
Exothermic reactions are best carried out in
-
What is the order of chemical reaction
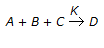 , if itis found that the reaction rate doubles on doubling the concentration of B and also the reaction rate doubles when the concentrations of both A & B were doubled and quandrupled when the concentrations of both B & C were doubled ?
, if itis found that the reaction rate doubles on doubling the concentration of B and also the reaction rate doubles when the concentrations of both A & B were doubled and quandrupled when the concentrations of both B & C were doubled ?
|
A.
A particular chemical reaction is more temperature sensitive at low temperatures.
|
|
B.
A very high value of equilibrium constant, K (K >> 1) indicates that the reaction is practically irreversible in nature.
|
|
C.
The intercept of the Arrhenious plot is called the 'activation energy'.
|
|
D.
Non-ideal flow takes place in reactors due to recycling, channeling or by creation of stagnant regions.
|


 Whatsapp
Whatsapp
 Facebook
Facebook