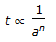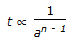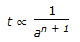Chemical Engineering :: Chemical Reaction Engineering
-
Reactions with high activation energy are
-
If n = overall order of a chemical reaction. a = initial concentration of reactant. t = time required to complete a definite fraction of the reaction. Then pick out the correct relationship.
-
In case of a/an __________ chemical reaction, conversion increases with the rise in temperature.
-
A space velocity of 5 hr-1 means that
-
With increase in initial concentration, the fractional conversion of a first order reaction in a given time
-
The rate of reaction of a/an __________ reaction is not affected by temperature rise.
-
With an increase in pressure in gaseous phase chemical reactions, the fractional conversion __________ when the number of moles decreases.
-
Half-life period for a first order reaction is __________ the initial concentration of the reactant.
-
Carbon particles accummulated on the catalyst used in the gas oil cracking lies in the category of __________ poison.
-
The decomposition of A into B is represented by the exothermic reaction, A
 2B. To achieve maximum decomposition, it is desirable to carry out the reaction.
2B. To achieve maximum decomposition, it is desirable to carry out the reaction.


 Whatsapp
Whatsapp
 Facebook
Facebook