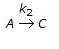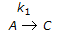Chemical Engineering :: Chemical Reaction Engineering
-
Pick out the wrong statement.
-
In a/an __________ reactor, there is exchange of heat with the surroundings with sizeable temperature variation.
-
'Unreacted core model' represents the reaction involving
-
For a vapour phase catalytic reaction (A + B
 P) which follows the Ridel mechanism and the reaction step is rate controlling, the rate of reaction is given by (reaction rate is irreversible, product also absorbs).
P) which follows the Ridel mechanism and the reaction step is rate controlling, the rate of reaction is given by (reaction rate is irreversible, product also absorbs). -
If a solid-gas non-catalytic reaction occurs at very high temperature, the rate controlling step is the __________ diffusion.
-
The units of frequency factor in Arhenious equation
-
Which of the following is not a chemical step in a fluid solid catalytic reaction ?
-
At a given temperature, K1, K2 and K3 are equilibrium constants for the following reactions 1, 2, 3 respectively.
CH4(g) + H2O(g) CO(g) + 3H2(g),
CO(g) + 3H2(g),
CO(g) + H2O(g) CO2(g) + H2(g)
CO2(g) + H2(g)
CH4(g) + 2H2O(g) CO2(g) + 4H2(g)
CO2(g) + 4H2(g)
Then K1, K2 and K3 are related as: -
In case of __________ reactions, the reaction rate does not decrease appreciably as the reaction proceeds.
|
A.
In a batch reactor, which is exclusively used for liquid phase reactions; temperature pressure and composition may vary with time.
|
|
B.
In a semi-batch reactor, one reactant is charged batchwise, while the other reactant is fed continuously.
|
|
C.
In a continuous flow reactor, uniform concentration can not be maintained throughout the vessel even in a well agitated system.
|
|
D.
In a continuous flow reactor, both the reactants and the products flow out continuously.
|


 Whatsapp
Whatsapp
 Facebook
Facebook



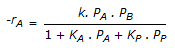



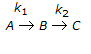 having k1 << k2, the reaction system can be approximated as
having k1 << k2, the reaction system can be approximated as