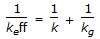Chemical Engineering :: Chemical Reaction Engineering
-
The reaction in which the rate equation corresponds to a stoichiometric equation, is called a/an __________ reaction.
-
A first order gaseous phase reaction is catalysed by a non-porous solid. The kinetic rate constant and the external mass transfer co-efficients are k and kg respectively. The effective rate constant (keff) is given by
-
Arrhenious equation represents graphically the variation between the __________ and temperature.
-
__________ catalytic reaction is involved in the thermal cracking of gas oil.
-
Variables affecting the rate of homogeneous reactions are
-
A chemical reaction occurs when the energy of the reacting molecules is __________ the activation energy of the reaction.


 Whatsapp
Whatsapp
 Facebook
Facebook