Chemical Engineering :: Chemical Reaction Engineering
-
A catalyst in a chemical reaction
-
Slurry reactors are characterised by the
-
Limiting reactant in a chemical reaction decides the
-
Consider the 'n' th order irreversible liquid phase reaction A
 B. Which one of the following plots involving half life of the reaction (t1/2) and the initial reactant concentration (CA0) gives a straight line plot ?
B. Which one of the following plots involving half life of the reaction (t1/2) and the initial reactant concentration (CA0) gives a straight line plot ? -
The rate of forward reaction, at chemical equilibrium is____the rate of backward reaction.
-
Pick the WRONG design guideline for a reactor in which the reactions, A
 R (desired) and A
R (desired) and A  S (undesired) are to take place. The ratio of the reaction rates is
S (undesired) are to take place. The ratio of the reaction rates is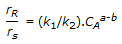 .
. -
With increase in temperature, the equilibrium __________ rises in case of endothermic reaction.
-
In case of a consecutive unimolecular type first order reaction
 , the concentration of component __________ increases continuously with time.
, the concentration of component __________ increases continuously with time. -
Enzymes (a protein) are catalysts found in organisms. Its efficiency of catalysing a reaction is due to its capacity to lower the activation energy of the reaction. The enzyme ptyalin used for food digestion is present in
-
Considering the endotheomic dissociation of CaCO3 in a closed vessel (CaCO3
 CaO + CO2), the pressure of CO2 increases, if
CaO + CO2), the pressure of CO2 increases, if


 Whatsapp
Whatsapp
 Facebook
Facebook


