Chemical Engineering :: Chemical Reaction Engineering
-
Half life period of decomposition of a liquid 'A' by irreversible first order reaction is 12 minutes. The time required for 75% conversion of 'A' is __________ minutes.
-
Decomposition rate of a liquid 'X' which decomposes as per the reaction
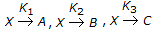 is given by
is given by -
With increase in the space time of an irreversible isothermal reaction being carried out in a P.F. reactor, the conversion will
-
A catalyst promoter
-
In case of a P.F.R., there
-
For the non-catalytic reaction of particles with surrounding fluid, the time needed to achieve the same fractional conversion for particles of different but unchanging sizes is proportional to the square of particle diameter, when the __________ is the controlling resistance.
-
If ΔG (free energy change) for a chemical reaction is very large and negative, then the reaction is
-
In a zero order reaction, reactants concentration does not change with time and the
|
A.
time for half change is half the time taken for completion of the reaction.
|
|
B.
time for half change is independent of the initial concentration.
|
|
C.
time for completion of the reaction is independent of the initial concentration.
|
|
D.
reaction rate is trebled when the initial concentration is trebled.
|


 Whatsapp
Whatsapp
 Facebook
Facebook


