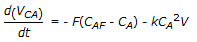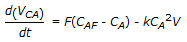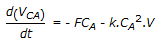Chemical Engineering :: Chemical Reaction Engineering
-
A first order reaction is to be treated in a series of two mixed reactors. The total volume of the two reactors is minimum, when the reactors are
-
A second order liquid phase reaction, A
 B, is carried out in a mixed flow reactor operated in semi batch mode (no exit stream). The reactant A at concentration CAF is fed to the reactor at a volumetric flow rate of F. The volume of the reacting mixture is V and the density of the liquid mixture is constant. The mass balance for A is
B, is carried out in a mixed flow reactor operated in semi batch mode (no exit stream). The reactant A at concentration CAF is fed to the reactor at a volumetric flow rate of F. The volume of the reacting mixture is V and the density of the liquid mixture is constant. The mass balance for A is -
Half life period of a chemical reaction is proportional to CA0-1 , if the reaction is of __________ order.
-
The irreversible reaction, X
 Y, is the special case of the reversible reaction, X
Y, is the special case of the reversible reaction, X  Y, in which the
Y, in which the -
The equilibrium constant K of a chemical reaction depends on
-
The reaction rate almost gets doubled for 10°C rise in temperature. This is due to the fact that the
-
Which of the following is not a unit of reaction rate ?
-
Brunaur, Emmet and Teller (BET) equation is used to determine the specific surface area of a porous particle but not the pore volume & the porosity of the catalyst bed. Which of the following postulates is not used to derive BET equation ?
-
The sequence in which three CSTR's of volumes 5, 10 and 15 m3 will be connected in series to obtain the maximum production in a second order irreversible reaction is
|
A.
Langmuir's assumption applies to every adsorbed layer.
|
|
B.
There is no dynamic equilibrium between successive layer.
|
|
C.
The adsorbed layer may be polymolecular in thickness and the heat of adsorption in each layer (except the first one) is involved in each of the evaporation process.
|
|
D.
none of these.
|


 Whatsapp
Whatsapp
 Facebook
Facebook