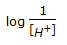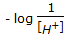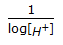Chemical Engineering :: Stoichiometry
-
Pick out the wrong unit conversion of temperature.
-
"The total volume occupied by a gaseous mixture is equal to the sum of the pure component volumes". This is the __________ law.
-
The osmotic pressure of a solution is directly proportional to the
-
Acidity or alkanity of a solution is expressed by its pH value, which is defined as (where, [H+] = hydrogen ion concentration in the solution).
-
Raoult's law is not applicable to the
-
A compound was found having nitrogen and oxygen in the ratio 28 gm and 80 gm respectively. The formula of the compound is
-
The atomic heat capacities of all solid elements __________ with decrease in temperature.
-
Raoult's law states that 'the equilibrium vapor pressure that is exerted by a component in a solution is proportional to the mole fraction of that component'. This generalisation is based on the assumption that the
-
The heats of vaporisation of CS2, C2H5OH &H2O are 26.8, 38.6 & 40.6 KJ/kg mole respectively. The order of decreasing inter-molecular forces in these liquids is
|
A.
sizes of the component molecules are approximately equal.
|
|
B.
attractive forces between like and unlike molecules are approximately equal.
|
|
C.
component molecules are non-polar and no chemical combination or molecular association between unlike molecules takes place in the formation of the solution.
|
|
D.
all (a), (b) & (c).
|

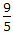 °F
°F 
 Whatsapp
Whatsapp
 Facebook
Facebook