Chemical Engineering :: Stoichiometry
-
Pick out the wrong statement.
-
Pick out the correct conversion.
-
The maximum adiabatic flame temperature of fuels in air is __________ the maximum flame temperature in pure oxygen.
-
Which of the following is not used for computing latent heat of vaporisation ?
-
The reverse process of fractional crystallisation is called
-
The unit of specific heat at constant pressure, Cp, in SI unit is
-
Two solutions A1 and A2 have pH value of 2 and 6 respectively. It implies that the solution
-
For water evaporating into usaturated air under adiabatic conditions and at constant pressure, the __________ remains constant throughout the period of vaporisation.
-
The molecular velocity of a real gas is proportional to (where, T = absolute temperature of the gas).
-
Pick out the wrong unit conversion of temperature.
|
A.
To make 100 kg of a solution containing 40% salt by mixing solution A (containing 25% salt) and solution B (containing 50% salt), the amount of solution A required is 40 kg.
|
|
B.
1.2 gm atoms of carbon and 1.5 gm moles of oxygen are reacted to give 1 gm mole of carbon dioxide. The limiting reactant is carbon. The percent excess reactant supplied is 25.
|
|
C.
A gas bubble at a pressure of Pg is passed through a solvent with a saturation vapour pressure of Ps. If the time of passage of the bubble is long and air is insoluble in the solvent, the mole fraction of solvent in the bubble will be equal to Ps/Pg.
|
|
D.
A supersaturated solution of a sparingly soluble solute, at a concentration of C, is being fed to a crystalliser at a volumetric flow rate of V. The solubility .of the solute is C1. The output rate of solids from an efficient crystalliser is (C + C1) V.
|


 Whatsapp
Whatsapp
 Facebook
Facebook



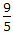 °F
°F