GATE 2017-2018 :: GATE Chemical
-
Consider the following set of linear algebraic equations x1 + 2x2 + 3x3 = 2x2 + x3 = - 12x2 + 2x3 = 0The system has
- If a and b are arbitrary constants, then the solution to the ordinary differential equation d2y/dx2 - 4y = 0 is
- For the function f(t) = e-t/T, the Taylor series approximation for t << T is
- A box containing 10 identical compartments has 6 red balls and 2 blue balls. If each compartment can hold only one ball, then the number of different possible arrangements are
-
Consider the following (2 * 2) matrix
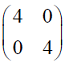 Which one of the following vectors is NOT a valid eigenvector of the above matrix ?
Which one of the following vectors is NOT a valid eigenvector of the above matrix ? - In a throttling process, the pressure of an ideal gas reduces by 50 %. If CP and CV are the heat capacities at constant pressure and constant volume, respectively (r = CP/CV) the specific volume will change by a factor of
- If the temperature of saturated water is increased infinitesimally at constant entropy, the resulting state of water will be
- In a parallel flow heat exchanger operating under steady state, hot liquid enters at a temperature Th, in and leaves at a temperature Th, out. Cold liquid enters at a temperature Tc, in and leaves at a temperature Tc, out. Neglect any heat loss from the heat exchanger to the surrounding. If Th, in >> Tc, in, then for a given time interval, which ONE of the following statements is true?
- For an exothermic reversible reaction, which one of the following correctly describes the dependence of the equilibrium constant ( K ) with temperature (T ) and pressure ( P ) ?
- Water is flowing under laminar conditions in a pipe of length L . If the diameter of the pipe is doubled, for a constant volumetric flow rate, the pressure drop across the pipe


 Whatsapp
Whatsapp
 Facebook
Facebook



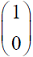 .
.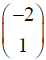 .
.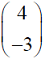 .
.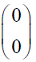 .
.