GATE 2017-2018 :: GATE Chemical
- The number of emails received on six consecutive days is 11, 9, 18, 18, 4 and 15, respectively. What are the median and the mode for these data?
- For two rolls of a fair die, the probability of getting a 4 in the first roll and a number less than 4 in the second roll, up to 3 digits after the decimal point, is _________
-
Which of the following statements are TRUE? P. The eigenvalues of a symmetric matrix are realQ. The value of the determinant of an orthogonal matrix can only be +1R. The transpose of a square matrix A has the same eigenvalues as those of AS. The inverse of an 'n × n' matrix exists if and only if the rank is less than 'n'
-
Evaluate
 ( Note: C is a constant of integration.)
( Note: C is a constant of integration.) -
A gaseous system contains H2, I2, and HI, which participate in the gas-phase reaction2 HI <-----> H2 + I2
At a state of reaction equilibrium, the number of thermodynamic degrees of freedom is _________ - The thermodynamic state of a closed system containing a pure fluid changes from (T1, p1) to (T2, p2), where T and p denote the temperature and pressure, respectively. Let Q denote the heat absorbed (> 0 if absorbed by the system) and W the work done (> 0 if done by the system). Neglect changes in kinetic and potential energies. Which one of the following is CORRECT?
- An equation of state is explicit in pressure p and cubic in the specific volume v. At the critical point 'c', the isotherm passing through 'c' satisfies
-
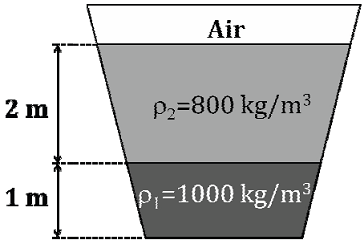
An open tank contains two immiscible liquids of densities (800 kg/m3 and 1000 kg/m3) as shown in the figure. If g = 10 m/s2, under static conditions, the gauge pressure at the bottom of the tank in Pa is ___________
- The apparent viscosity of a fluid is given by 0.007 |dV/dy|0.3 where (dV/dy) is the velocity gradient. The fluid is


 Whatsapp
Whatsapp
 Facebook
Facebook



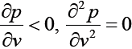 .
.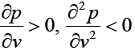 .
.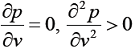 .
. .
.