GATE 2017-2018 :: GATE Chemical
- The exit age distribution for a reactor is given by E(t) = δ(t - 4), where t is in seconds. A first order liquid phase reaction (k = 0.25 s-1) is carried out in this reactor under steady state and isothermal conditions. The mean conversion of the reactant at the exit of the reactor, up to 2 digits after the decimal point, is __________
- An isothermal liquid phase zero order reaction A --> B (k = 0.5 mol/m3 -s) is carried out in a batch reactor. The initial concentration of A is 2 mol/m3. At 3 seconds from the start of the reaction, the concentration of A in mol/m3 is __________
- The overall rates of an isothermal catalytic reaction using spherical catalyst particles of diameters 1 mm and 2 mm are rA1 and rA2 (in mol (kg-catalyst)-1 h-1), respectively. The other physical properties of the catalyst particles are identical. If pore diffusion resistance is very high, the ratio rA2/rA1 is __________
- In the manufacture of sulphuric acid by the contact process, the catalytic oxidation of SO2 is carried out in multiple stages mainly to
-
Match the following.Group 1 Group 2(P) Viscosity (1) Pyrometer(Q) Pressure (2) Hot wire anemometer(R) Velocity (3) Rheometer(S) Temperature (4) Piezoelectric element
- For the function f(z) = 1/[(2 - z)(z + 2] the residue at z = 2 is _________
- The solution of the differential equation dy/dx - y2 = 0, given y = 1 at x = 0 is
- The solution of the differential equation d2y/dx2 - dy/dx + 0.25y = 0, given y = 0 at x = 0 and dy/dx = 1 at x = 0 is
-
The value of the integral
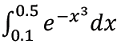 evaluated by Simpson's rule using 4 subintervals (up to 3 digits after the decimal point) is ____
evaluated by Simpson's rule using 4 subintervals (up to 3 digits after the decimal point) is ____ - In a process occurring in a closed system F, the heat transferred from F to the surroundings E is 600 J. If the temperature of E is 300 K and that of F is in the range 380 - 400 K, the entropy changes of the surroundings (ΔSE) and system (ΔSF), in J/K, are given by


 Whatsapp
Whatsapp
 Facebook
Facebook


